Introduction
Towards a more sustainable European pharmaceutical production of API's
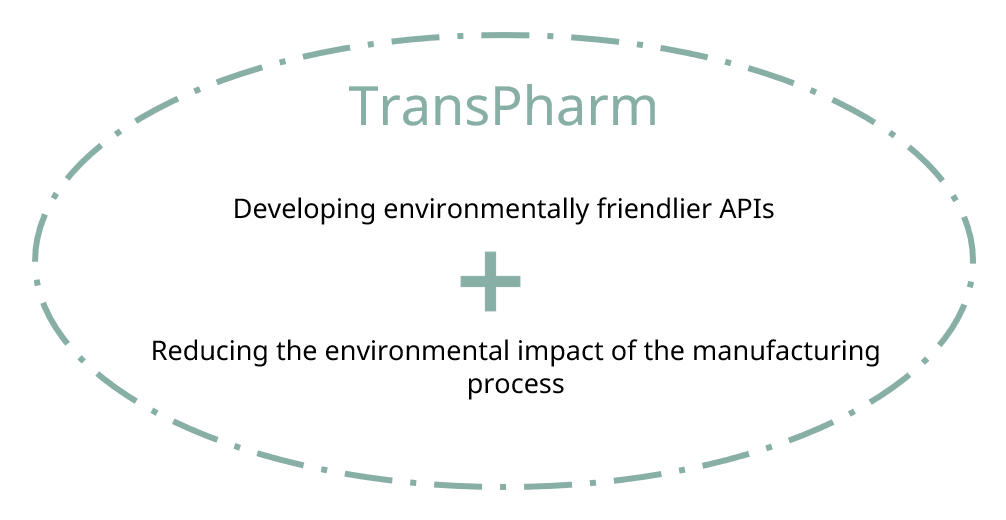
Pharmaceuticals comprise a wide variety of chemical compounds designed to guarantee safe and effective therapies. The active pharmaceutical ingredients (APIs) are those that deliver the beneficial health effects experienced by the patients. The goal of the project is to develop more sustainable and greener APIs that simultaneously reduce the environmental footprint and the dependence on third countries for API production. The EU-funded TransPharm project will deliver digital tools and guidelines, also based on artificial intelligence, for the development of greener pharmaceutical products and APIs, as well as models to judge their impact. The result will be a more independent and competitive European pharmaceutical industry, which can ensure the timely delivery of sustainable and green therapeutics.
|
Duration 48 months |
Budget € 8M |
11 Partners / 8 EU countries Belgium, The Netherlands, Germany, Norway, Finland, Ireland, France and UK. |
|
Objectives
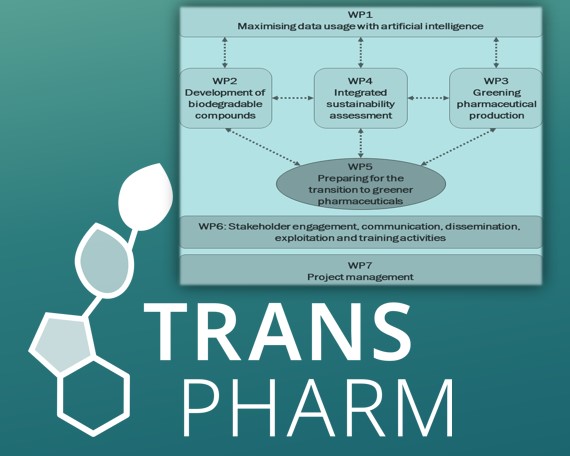
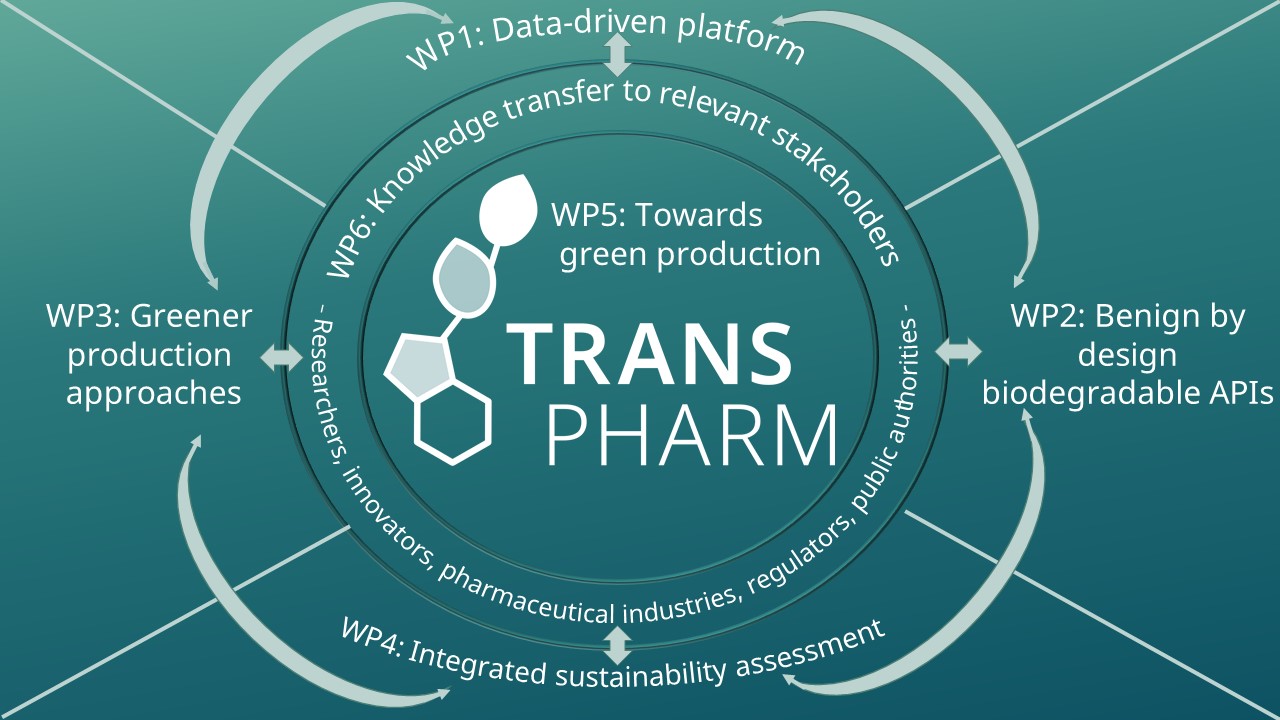
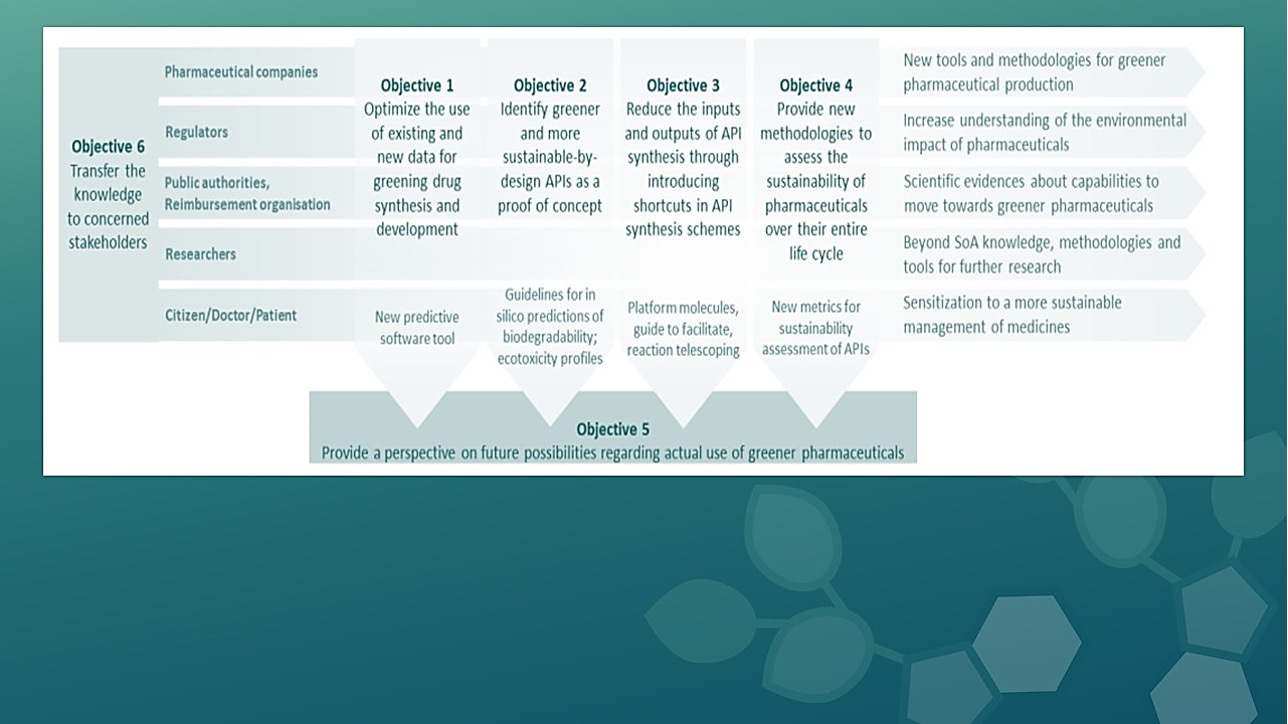
TransPharm is a Horizon Europe project, coordinated by Ghent University in Belgium, and aims to move towards a sustainable pharmaceutical industry and to improve the European readiness for sustainable production of small molecules (active pharmaceutical ingredients, APIs) that have environmental or supply concerns. To reach the envisaged aims, the project will deliver four toolboxes for the development of greener pharmaceutical products and APIs, with the aim of analysing and predicting flow behaviour and environmental biodegradability and ecotoxicity of APIs and their synthesis pathways, identifying greener and more sustainable alternatives to pharmaceutical products / APIs of concern, reducing the footprint in synthetic schemes of APIs, and assessing the sustainability of pharmaceuticals over their entire life cycle. These toolboxes will be used to assess the potential to move towards the transition to greener pharmaceutical production. In addition, the team will elaborate on business cases for sustainable pharmaceutical products or APIs and what is needed to bring them to the market. The project will also make sure that key project results and knowledge are properly transferred towards targeted stakeholders.
Expected impacts
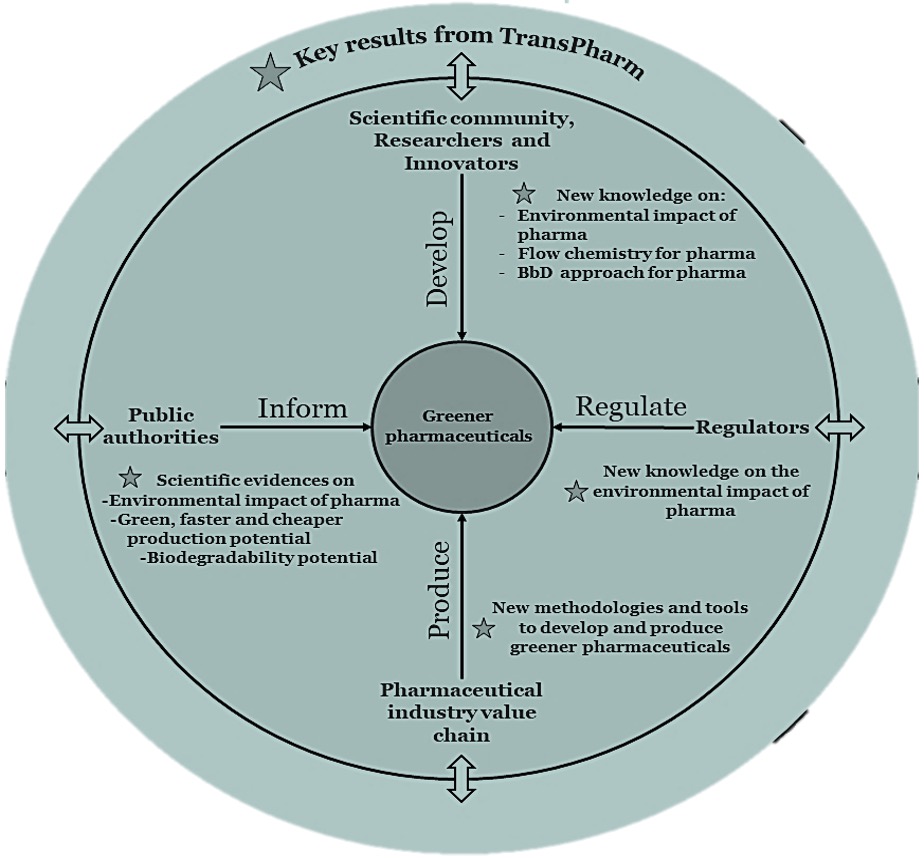
The TransPharm project’s objective is to share the knowledge with relevant target groups so that the results can be easily exploited by the healthcare value chain.
- The project will propose a multi-level approach to provide researchers and regulators with a better understanding of the environmental impact of pharmaceuticals. Tools and guidelines will be developed and shared with key stakeholders, so that projects key results are widely known and adopted.
These project’s key results and knowledge will be transferred to targeted stakeholders: beyond researchers and regulators, it is also of high importance that a wider community understands the environmental impact of pharmaceuticals. - The project’s objective is to generate scientific evidence related to the development, production, use and disposal of pharmaceuticals, so that public authorities can inform pharmaceutical strategies and policies based on scientific evidence.
- The project will contribute to the development and future use of greener pharmaceuticals by
(i) Creating a centre of excellence, with training actions dedicated to the young generation and researchers;
(ii) Provide scientific evidence that might help to the development of new standards
(iii) Providing scientific evidence so that public authorities can inform pharmaceutical strategies and polices
(iv) Empowering the healthcare sector and general public to a more sustainable use of pharmaceuticals, including sustainable procurement of pharmaceuticals.
These elements combined to the development of new methodologies, tools and guidelines for the techno-economic transition to greener pharmaceuticals, the project will have an impact on all factors enabling this transition.
It will assure reliable access to key manufacturing capacity and will bring back production to Europe. The EU will create an autonomous leadership position with on demand, faster, cheaper and more sustainable manufacturing of medicines products.
In a longer term, the uptake of methodologies and tools are envisioned to have a significant impact in reducing carbon footprint of pharmaceutical production, while reducing the presence of harmful substance in the environment.
The project will contribute to  and
and  .
.
Workplan
The TransPharm project is articulated around seven Work Packages.